First Patient Begins Newly Approved Sickle Cell Gene Therapy
Source: Webmd.com
Incident Overview:
- Kendric Cromer, a 12-year-old with sickle cell disease, has begun a new genetic treatment outside of clinical trials.
- The treatment, shown to cure 88% of people with sickle cell disease, offers hope to the estimated 100,000 individuals in the U.S. affected by the condition, which is more common among Black people.
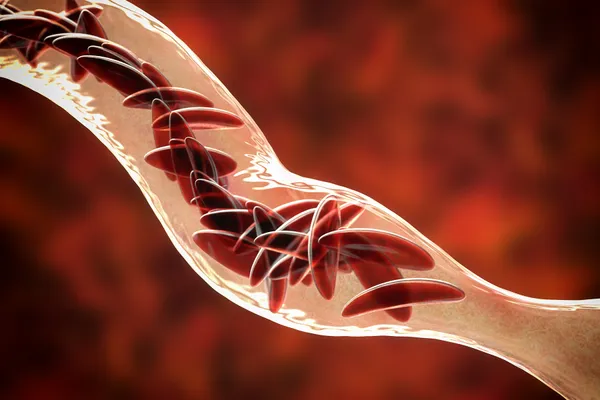
- The therapy, called Lyfgenia, involves a months-long process beginning with the collection of bone marrow stem cells at Children’s National Hospital in Washington, DC.
- Bluebird Bio, the company behind the treatment, has set a price tag of $3.1 million for the therapy, making it one of the most expensive medical treatments, with a current annual capacity to treat between 85 and 105 patients.
- The modified stem cells are designed to counteract the effects of mutated proteins causing sickle-shaped red blood cells, aiming to restore normal red blood cell production and function.
Conclusion:
In conclusion, Kendric Cromer’s groundbreaking journey as the first person with sickle cell disease to undergo a commercially approved gene therapy offers hope and potential life-changing benefits to thousands of individuals facing this debilitating condition. The successful implementation of this treatment highlights the progress in medical advancements and underscores the importance of continued research and innovation in improving the lives of those affected by genetic disorders. Kendric’s story serves as a beacon of hope for the future of sickle cell disease treatment and inspires optimism for a better quality of life for patients worldwide.
To read more click link: https://childreninfobank.com/safebank/first-patient-begins-newly-approved-sickle-cell-gene-therapy/
Source of image: webmd.com





